Last Updated: August 2023
PROJECT DESCRIPTION
BACKGROUNDImpregnation
of
drugs
into
a
porous
carrier
can
improve
some
crucial
properties
of
the
drugs
such
as
its
dissolution
behavior,
content
uniformity,
and
flow
properties.
Therefore,
several
techniques
have
been
developed
for
loading
crystalline
drugs
into
porous
carriers.
However,
most
of
these
methods
show
many
disadvantages
and
manufacturing
problems.
Therefore,
it
is
of
interest
to
introduce
a
pharmaceutical
manufacturing
method
that
can
overcome
the
drawbacks
of
previous
loading
methods. Also, it is important to study how this method can facilitate the drug and product development.
PROJECT GOALS
The
main
goals
of
this
project
are
to
show
the
applicability
of
fluidized
bed
impregnation
technique
and
the
advantages
of
this
technique
over
other
loading
methods.
Also,
the
improvement
in
properties
of
the
drugs
after
impregnation
into
porous
have been studied to present the advantages of incorporating drugs into porous carriers.
SUMMARY OF STUDIES
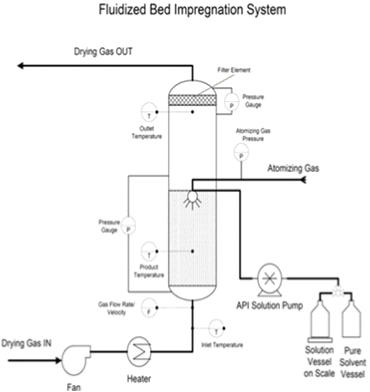
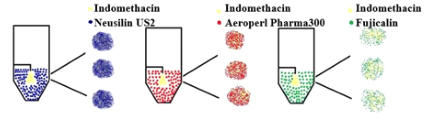
In this work, the essential steps to successfully loading drugs into porous carriers have been developed. The main procedure steps include: • Preparation of API solution. • Loading porous carrier into the fluidized bed. The mass of porous carrier should be enough to reach the top spray nozzle. • Fluidizing the porous carrier using only hot air. • Spraying only solvent until reaching the steady state. Then, spraying API solution. After achieving the required loading, switching the spray back into pure solvent. • Drying and collecting the samples for further characterization tests, which include blend uniformity, flow properties, X-ray diffraction, differential scanning calorimetry, surface area and pore volume, and dissolution behavior. Figure 1 describes the required set up steps to successfully load drugs into porous carriers. Figure 1. The experimental set up of fluidized bed impregnation method To show the generality and robustness of this technique, the effects of drug loading and solvent types have been studied by loading acetaminophen into Neusilin using water and methanol as solvents. The impregnated product present acceptable blend uniformity, excellent flow properties, and a narrow particle size distribution. Also, the results suggest that the improvement in the properties of impregnated products does not depend on the drug loading or solvent type. Moreover, to explore the generality of fluidized bed impregnation method, the effect of carrier type on the properties of impregnated has also been investigated. To achieve this goal, Indomethacin (IMN) was loaded into Neusillin (NEU), Aeroperil (AER) and Fujicalin (FUJ). The results show that IMN has successfully impregnated into NEU, AER and FUJ. The results also demonstrate that the impregnated products generally have good properties, which are important for manufacturing of solid dosage forms. Figure 2 represents the schematic diagram of loading IMN into three different carriers. Figure 2. Loading of Indomethacin into three different carriers using fluidized bed dryer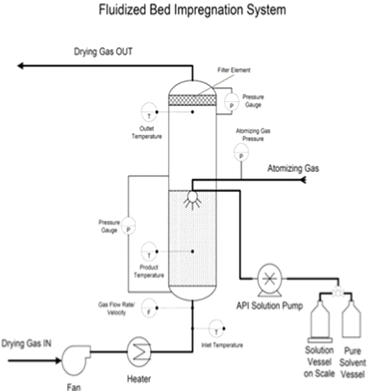
Last Updated: August 2023
PROJECT DESCRIPTION
BACKGROUNDImpregnation
of
drugs
into
a
porous
carrier
can
improve
some
crucial
properties
of
the
drugs
such
as
its
dissolution
behavior,
content
uniformity,
and
flow
properties.
Therefore,
several
techniques
have
been
developed
for
loading
crystalline
drugs
into
porous
carriers.
However,
most
of
these
methods
show
many
disadvantages
and
manufacturing
problems.
Therefore,
it
is
of
interest
to
introduce
a
pharmaceutical
manufacturing
method
that
can
overcome
the
drawbacks
of
previous
loading
methods.
Also,
it
is
important
to
study
how
this
method can facilitate the drug and product development.
PROJECT GOALS
The
main
goals
of
this
project
are
to
show
the
applicability
of
fluidized
bed
impregnation
technique
and
the
advantages
of
this
technique
over
other
loading
methods.
Also,
the
improvement
in
properties
of
the
drugs
after
impregnation
into
porous
have
been
studied
to
present
the
advantages
of
incorporating
drugs
into
porous carriers.
SUMMARY OF STUDIES
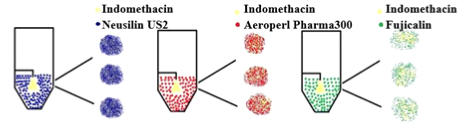
In this work, the essential steps to successfully loading drugs into porous carriers have been developed. The main procedure steps include: • Preparation of API solution. • Loading porous carrier into the fluidized bed. The mass of porous carrier should be enough to reach the top spray nozzle. • Fluidizing the porous carrier using only hot air. • Spraying only solvent until reaching the steady state. Then, spraying API solution. After achieving the required loading, switching the spray back into pure solvent. • Drying and collecting the samples for further characterization tests, which include blend uniformity, flow properties, X-ray diffraction, differential scanning calorimetry, surface area and pore volume, and dissolution behavior. Figure 1 describes the required set up steps to successfully load drugs into porous carriers. Figure 1. The experimental set up of fluidized bed impregnation method To show the generality and robustness of this technique, the effects of drug loading and solvent types have been studied by loading acetaminophen into Neusilin using water and methanol as solvents. The impregnated product present acceptable blend uniformity, excellent flow properties, and a narrow particle size distribution. Also, the results suggest that the improvement in the properties of impregnated products does not depend on the drug loading or solvent type. Moreover, to explore the generality of fluidized bed impregnation method, the effect of carrier type on the properties of impregnated has also been investigated. To achieve this goal, Indomethacin (IMN) was loaded into Neusillin (NEU), Aeroperil (AER) and Fujicalin (FUJ). The results show that IMN has successfully impregnated into NEU, AER and FUJ. The results also demonstrate that the impregnated products generally have good properties, which are important for manufacturing of solid dosage forms. Figure 2 represents the schematic diagram of loading IMN into three different carriers. Figure 2. Loading of Indomethacin into three different carriers using fluidized bed dryer